Optimizing MAP Targets in Septic Shock: Insights from the OPTPRESS Trial

Get concise anesthesia & Critical Care updates, every week.
Join the growing community of anesthesiologists and intensivists staying current on the latest evidence.
1- Introduction:
When managing septic shock, particularly in older patients with chronic hypertension, a critical decision arises: what Mean Arterial Pressure (MAP) target should guide vasopressor therapy? While the Surviving Sepsis Campaign (SSC) typically recommends a 65 mmHg MAP, the optimal target for this complex patient population remains debated. The OPTPRESS trial (Efficacy of targeting high mean arterial pressure for older patients with septic shock) was specifically designed to investigate whether a higher MAP target could benefit elderly patients with hypertension in septic shock.
2- Research Methodology:
2.1- Study Design:
The OPTPRESS trial was conducted as a multicenter, pragmatic, open-label, randomized controlled trial across 29 hospitals in Japan. While treating physicians were aware of group assignments, statisticians remained blinded to allocation until all analyses were complete, ensuring analytical objectivity.
2.2- Participant Eligibility: Defining the Study Population

2.3- Intervention Protocol:
Patients were randomized 1:1 to one of two distinct hemodynamic management strategies:
- Group Assignment:
- High-Target Group: Aimed for a Mean Arterial Pressure (MAP) of 80–85 mmHg.
- Control Group: Targeted a MAP of 65–70 mmHg, aligning with the 2021 Surviving Sepsis Campaign (SSC) guidelines.
- MAP Target Duration:
- Target MAPs were maintained for up to 72 hours post-randomization or until vasopressor support was no longer clinically required.
- Beyond 72 hours, the specific MAP target was determined at the treating physician's discretion!!
- Blood Pressure Monitoring:
- Primarily relied on non-invasive measurements.
- Invasive arterial pressure monitoring was utilized when non-invasive methods were impractical.
- High-Target Group Safety Measures:
- If vasopressor-related adverse events occurred (such as significant bleeding, myocardial infarction, severe arrhythmias, intestinal ischemia, or limb ischemia), the MAP target in the high-target group was reduced to 65 mmHg.
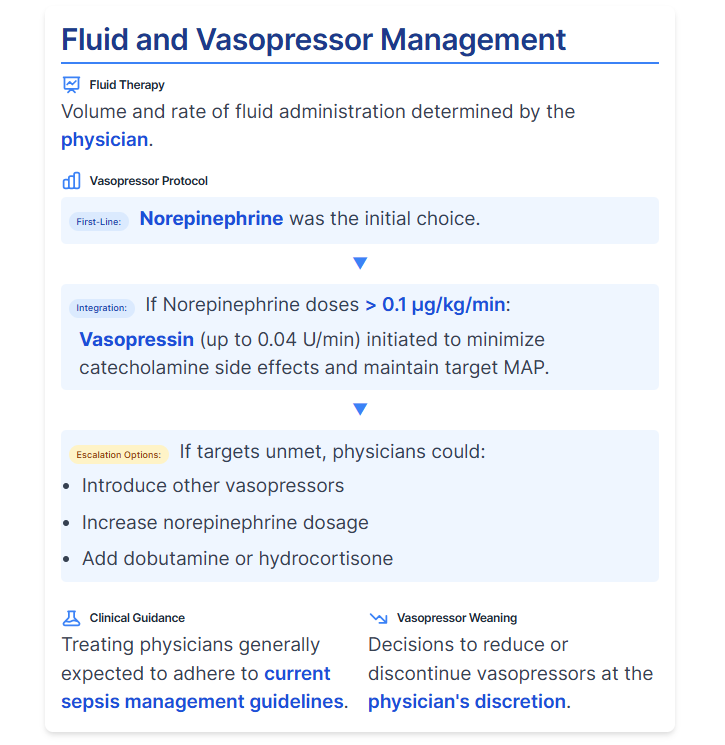
- Fluid and Vasopressor Management:
- Fluid Therapy: Volume and rate of fluid administration were determined by the physician.
- First-Line Vasopressor: Norepinephrine was the initial choice.
- Vasopressin Integration: To minimize catecholamine side effects, vasopressin (up to 0.04 U/min) was initiated if norepinephrine doses exceeded 0.1 μg/kg/min to maintain target MAP.
- Escalation Options: If targets remained unmet, physicians could introduce other vasopressors, increase norepinephrine dosage, or add dobutamine or hydrocortisone.
- Clinical Guidance: Treating physicians were generally expected to adhere to current guidelines for sepsis management.
- Vasopressor Weaning: Decisions to reduce or discontinue vasopressors were at the physician's discretion.
- Ancillary Clinical Management:
- Antibiotic selection, mechanical ventilation, renal replacement therapy, and other medical interventions were managed at the physician's discretion.
- Sedation and pain management aimed for a Richmond Agitation-Sedation Scale (RASS) score between -3 and 0.


2.4- Outcome Measures: Assessing Efficacy and Safety
The trial utilized both primary and secondary endpoints to comprehensively evaluate the impact of the different MAP strategies.
- Primary Outcome:
- All-cause mortality at 90 days following randomization. This was confirmed by the treating physician via medical records, direct patient/relative contact, or communication with transfer facilities.
- Secondary Outcomes:
- Mortality Rates:
- All-cause mortality at 28 days.
- Sepsis-related mortality at 28 days.
- Sepsis-related mortality at 90 days.
- Lactate clearance at 24 hours.
- Safety outcomes:
- Mortality Rates:
- Incidence of arrhythmias (ventricular tachycardia or hemodynamically significant supraventricular arrhythmia) within 72 h.
- Incidence of thromboembolic events (e.g., myocardial infarction, cerebral infarction, intestinal necrosis, irreversible peripheral limb ischemia) within 72 h.
- Incidence of hemorrhagic events within 72 h.
- Organ Support-Free Days at 28 Days: (For these measures, patients who died within 28 days or remained on support after 28 days were assigned a score of zero.)
- Ventilator-free days.
- Renal replacement therapy (RRT)-free days.
- Catecholamine-free days.
- Measurement Protocols:
- Adverse events like arrhythmias or thromboembolism were clinically assessed by the physician using laboratory data, imaging, and other relevant findings.
- "Free days" were defined as consecutive 24-hour periods from day 1 to day 28 post-randomization where the patient was alive and did not require the specified support.
3- Results: Unveiling the Impact of MAP Targets
The trial's findings provided critical insights into the safety and efficacy of high-target MAP strategies in this patient population.
- Participant Flow: A total of 518 patients were enrolled and analyzed, with 258 assigned to the high-target group and 260 to the control group.
- Baseline Comparability: Patient baseline characteristics were well-balanced between both the high-target and control groups.
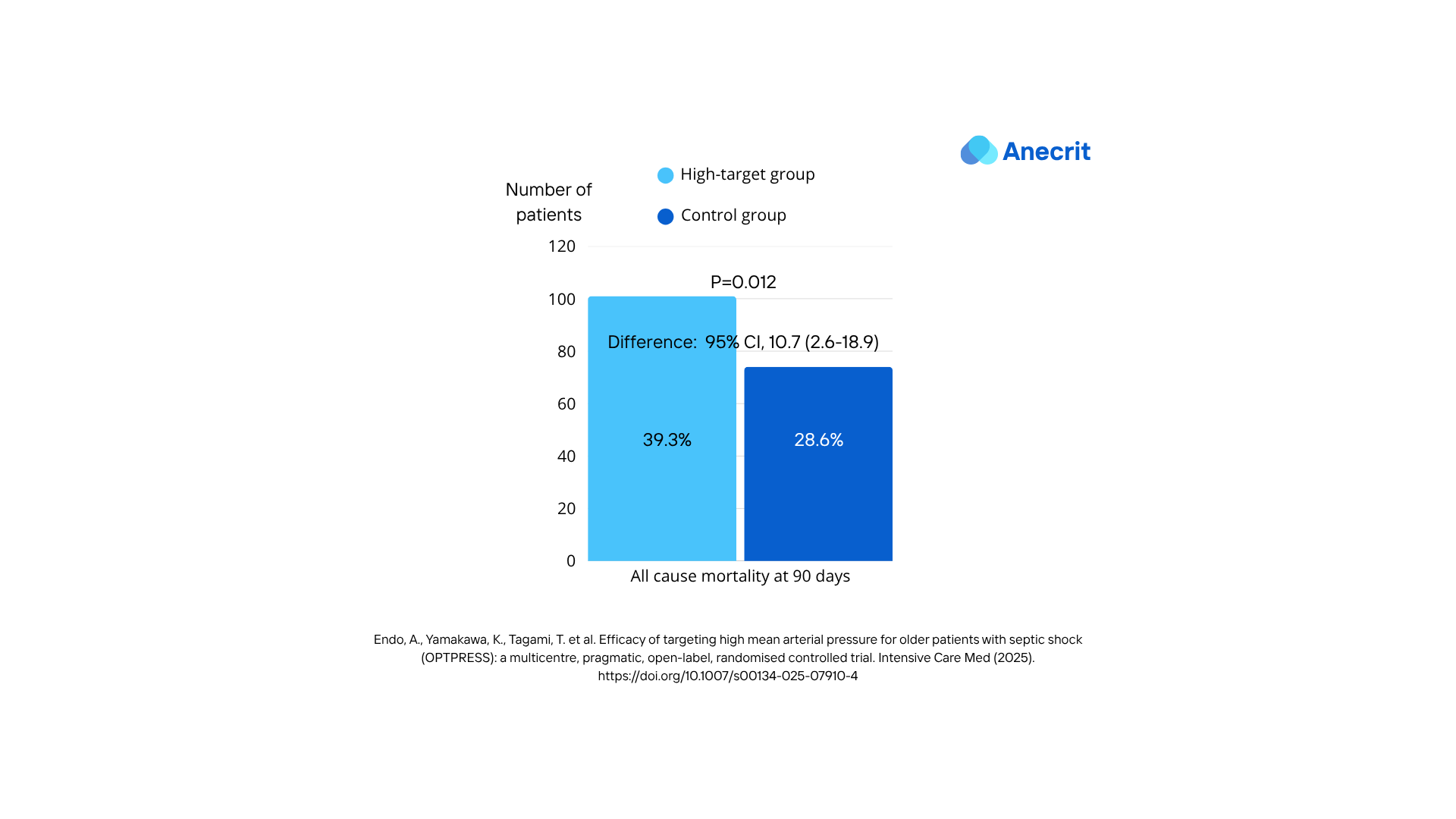
- Primary Outcome Findings: The trial was prematurely concluded following an interim analysis, which indicated potential harm associated with the high-target MAP strategy. At 90 days post-randomization, all-cause mortality was significantly higher in the high-target group (39.3%, 101 patients) compared to the control group (28.6%, 74 patients). This resulted in a risk difference of 10.7 (95% confidence interval, 2.6–18.9), demonstrating a statistically significant increase in mortality with the higher MAP target.
- Secondary Outcomes Findings:
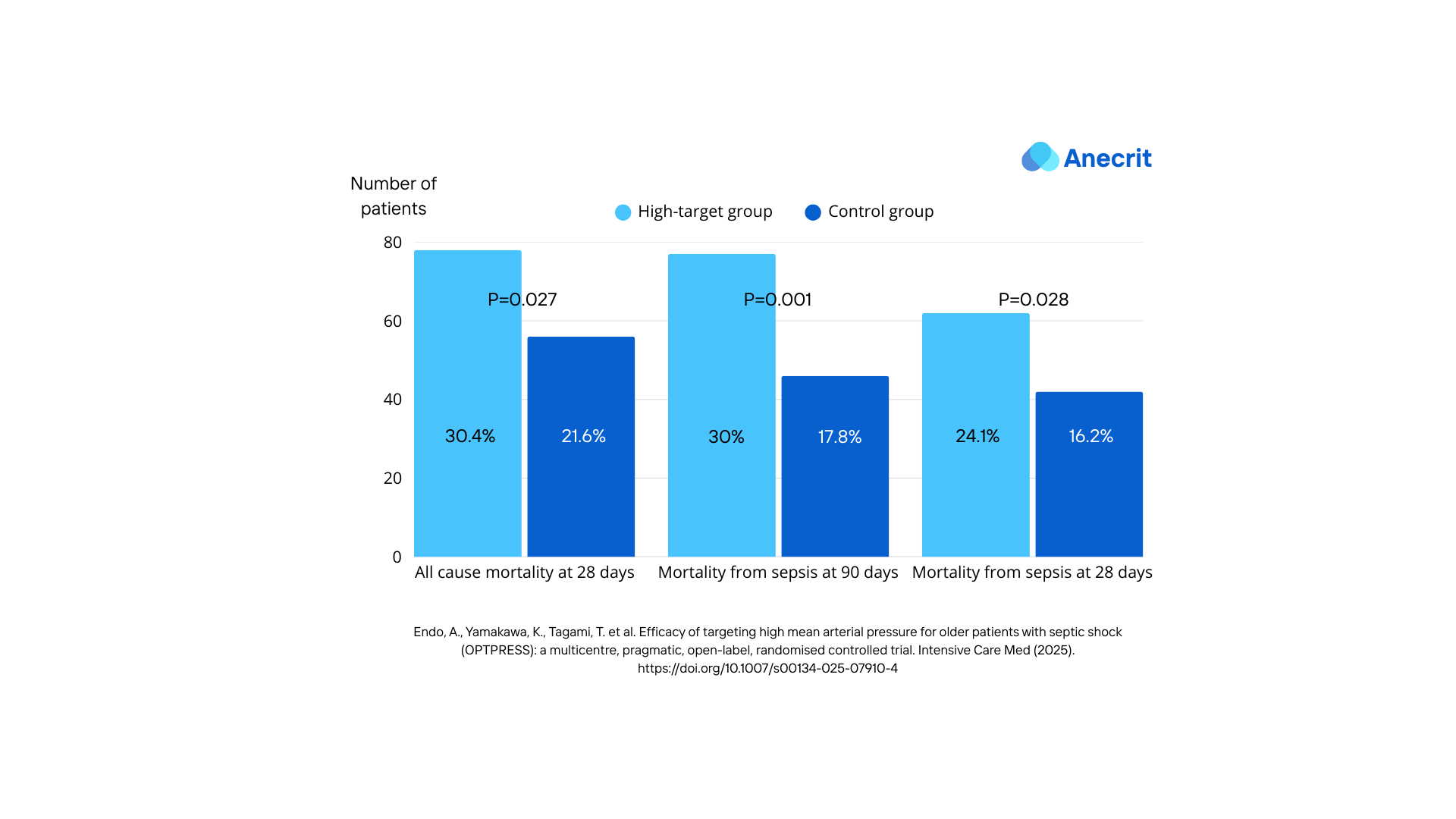
- All mortality rates from the secondary outcomes were higher in the high target group.
- All organ support-free days outcomes were lower in the high target group.
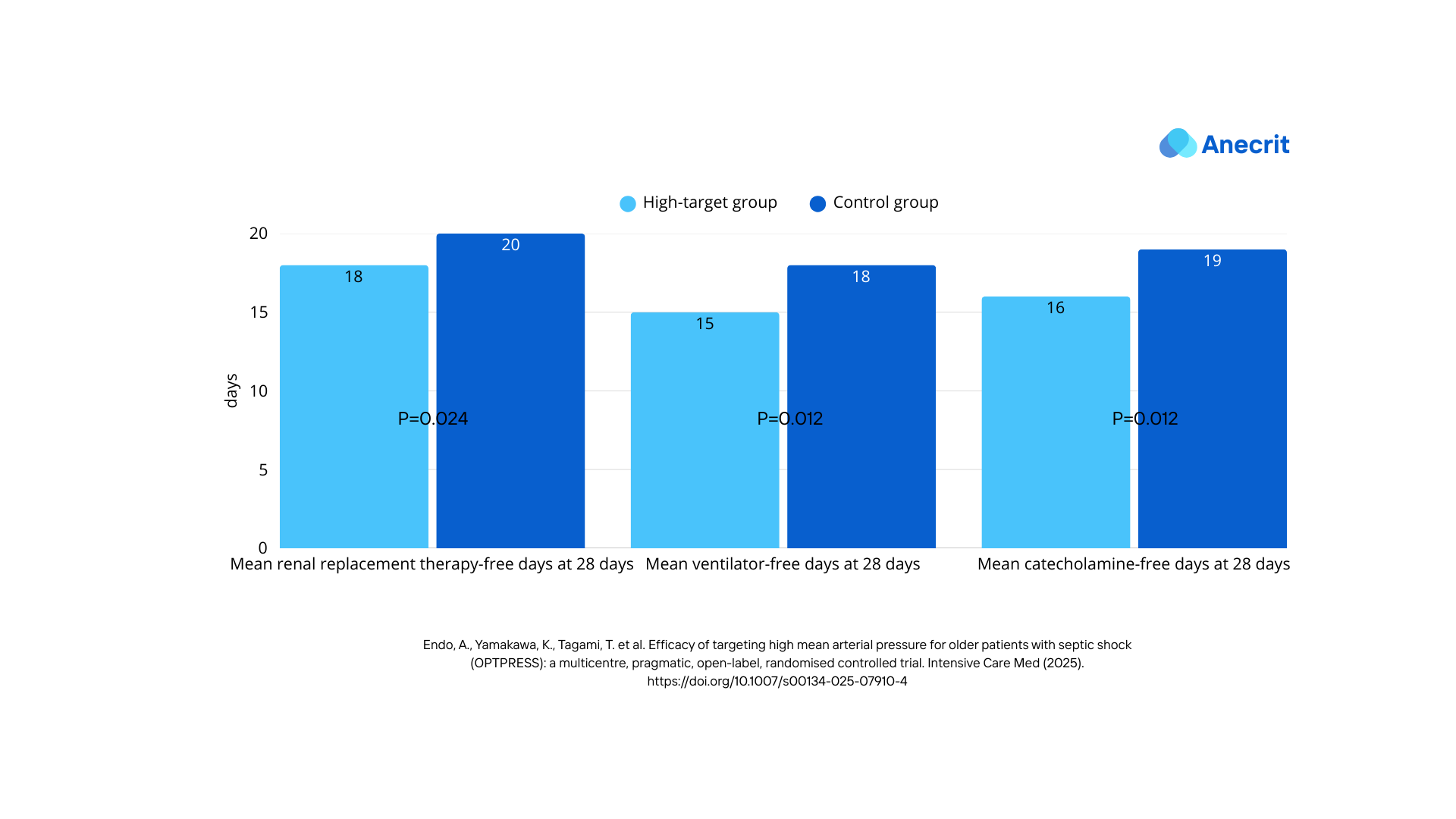
- Adverse Events/Side Effects: While the published results did not find a statistically significant difference in safety outcomes, the early termination of the study could be the cause for that. (It's worth mentioning that the target MAP was reduced to 65- 70 mmHg in two patients in the high target group due to adverse events)
4- Strengths of the Study Design and Execution
- Multicenter, Pragmatic, Randomized Controlled Trial: The study design is robust for evaluating clinical effectiveness in a real-world setting. Conducting it across 29 hospitals in Japan enhances its practical relevance.
- Targeted Underrepresented Population: The study specifically focused on older patients (aged ≥ 65 years) with septic shock in Japan, a population and region previously underrepresented in similar trials, thereby adding global relevance to existing evidence.
- Clear Primary Outcome: The primary outcome of 90-day all-cause mortality is clinically relevant and objective.
- Randomization and Stratification: Patients were randomized 1:1 using a centralized, computer-generated allocation sequence, stratified by history of chronic hypertension and age (<80 years or older) to ensure balanced groups at baseline. Baseline characteristics were indeed similar between the groups.
- Early Termination Based on Harm: The trial's early termination due to an interim analysis suggesting harm in the high-target strategy demonstrates ethical conduct and responsiveness to patient safety.
- Protocol for Vasopressin Use: The protocol included early concomitant use of vasopressin to minimize potential adverse effects of catecholamines.
- Intention-to-Treat Analysis: The primary analysis was conducted on an intention-to-treat population, which maintains the benefits of randomization and provides a more pragmatic estimate of the intervention's effect.
- Masked Statisticians: The statisticians were masked to group allocation until all analyses were completed, reducing analytical bias.
5- Potential Limitations/Weaknesses:
- Open-Label Design: The trial was open-label, meaning treating physicians were aware of the assigned groups. This could influence clinical management and introduce performance bias.
- Non-Invasive Blood Pressure Monitoring: Blood pressure was measured non-invasively on the upper arm in principle for generalizability, but invasive intra-arterial blood pressure monitoring, which is more accurate, is typically feasible in ICU settings and might have provided more precise MAP control.
- Physician Discretion for Certain Treatments: Several aspects of patient management, including fluid administration volume and speed, vasopressor reduction/discontinuation, initial empiric antibacterial agents, mechanical ventilation/RRT introduction, and use of other adjunctive medications, were left to the treating physician's discretion. While this contributes to the pragmatic nature, it could introduce variability in care.
- Lack of Strict Vasopressor Titration Protocol: The absence of a strict titration or tapering protocol for vasopressors might have led to differences in the time to reach target MAP and the accuracy of MAP control.
- Cardiac Function Monitoring Not Protocolized: Cardiac function monitoring and management were not protocolized, despite the acknowledgement that the effectiveness of fluid resuscitation and vasoactive agents depends on pre-existing cardiovascular conditions and cardiac depression due to sepsis. This could be a significant uncontrolled variable.
- Difference Between Target and Observed MAP in Control Group: The discussion acknowledges that the actual MAP in the control group often exceeded the target range (65-70 mmHg), though it attributes this primarily to improved patient condition rather than protocol non-compliance. However, this could still potentially blur the distinction between the two intervention arms, especially if the "excess" MAP in the control group approached the lower end of the high-target group's range.
- Generalizability of the Findings:
- Single-Country and Ethnic Homogeneity: The study was conducted in 29 hospitals across Japan, and all participants were Japanese. This limits the generalizability of the findings to other ethnic groups and healthcare systems outside of Japan.
- Specific Patient Population (Older Patients with Septic Shock): The findings are specific to older patients (aged ≥ 65 years) with septic shock. While this was a strength in addressing an underrepresented population, it means the results may not be directly applicable to younger septic shock patients.
6- Question arises:
The OPTPRESS trial truly reinforces the current Surviving Sepsis Campaign guidelines regarding MAP targets. But this outcome naturally leads us to wonder: why these specific results?
From a pathophysiological standpoint, a higher MAP target might intuitively seem more appropriate for hypertensive patients with septic shock, yet this trial showed increased mortality in that group. This raises a compelling question about the underlying reasons. Could it be linked to adverse effects from catecholamine use? While the trial didn't find a statistically significant difference in adverse events, the study's early termination could be a factor here. Perhaps a completed trial would have revealed a clearer statistical significance regarding these adverse events.
I'll aim to delve deeper into this question in an upcoming article. If you're interested in not missing it, be sure to subscribe to get notified upon publication!
Feedback & Suggestions
Don't hesitate to share your thoughts and suggestions in the comments below.
References:
- Endo, A., Yamakawa, K., Tagami, T. et al. Efficacy of targeting high mean arterial pressure for older patients with septic shock (OPTPRESS): a multicentre, pragmatic, open-label, randomised controlled trial. Intensive Care Med 51, 883–892 (2025).
- Evans, L., Rhodes, A., Alhazzani, W. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47, 1181–1247 (2021).
Keywords:
septic shock, MAP targets, Mean Arterial Pressure, older patients, vasopressors, OPTPRESS trial, randomized controlled trial, sepsis guidelines, mortality in sepsis, hemodynamic management, vasopressor therapy, sepsis treatment, clinical trial results, intensive care unit, sepsis mortality, Surviving Sepsis Campaign, norepinephrine, vasopressin, sepsis research, medical research, critical care medicine